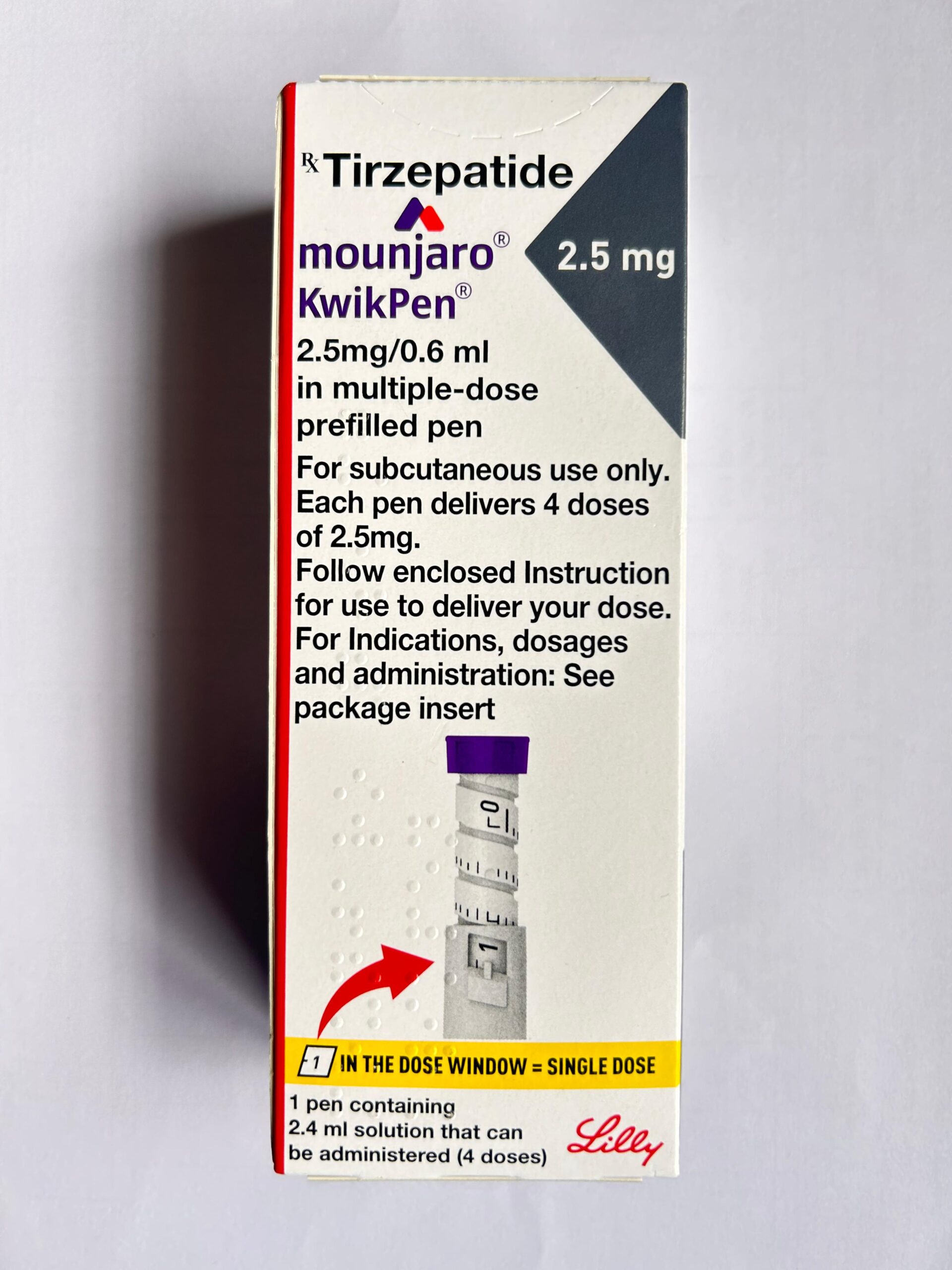
Mounjaro 2.5 mg KwikPen Injection contains Tirzepatide, a novel once-weekly injectable medicine used for the management of Type 2 Diabetes Mellitus. Tirzepatide is a dual-acting incretin mimetic that targets both GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors.
The 2.5 mg dose is generally prescribed as a starting dose to allow gradual dose escalation and improved tolerability. Mounjaro helps improve blood sugar control by enhancing insulin secretion, reducing glucagon levels, slowing gastric emptying, and regulating appetite.
Uses of Mounjaro 2.5 mg Injection
-
Management of Type 2 Diabetes Mellitus
-
Improvement of glycemic control along with diet and exercise
-
Initial dose for treatment initiation
-
Helps control fasting and post-meal blood glucose levels
How Mounjaro Works (Mechanism of Action)
Tirzepatide activates both GIP and GLP-1 receptors, leading to:
-
Increased glucose-dependent insulin release
-
Reduced glucagon secretion
-
Delayed gastric emptying
-
Reduced appetite and calorie intake
These effects work together to improve overall glycemic control.
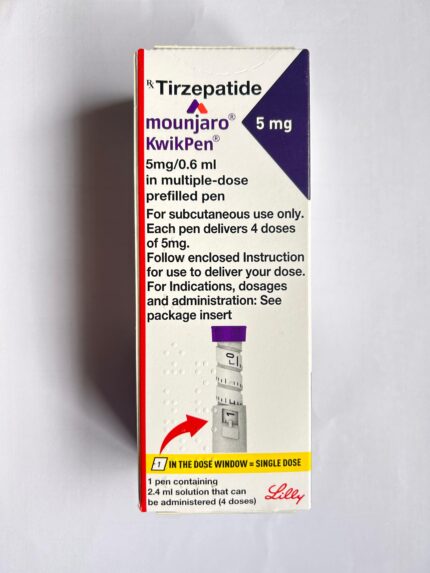
Dosage and Administration
-
Route: Subcutaneous injection (abdomen, thigh, or upper arm)
-
Starting Dose: 2.5 mg once weekly
-
Each pen delivers 4 weekly doses
-
Administer on the same day each week, with or without food
-
Follow the instructions provided in the package insert
Dosage adjustments should be done only under medical supervision.
Side Effects
Common Side Effects:
-
Nausea
-
Vomiting
-
Diarrhea
-
Decreased appetite
-
Constipation
-
Injection site reactions
Serious (Rare) Side Effects:
-
Severe gastrointestinal symptoms
-
Hypoglycemia (when used with insulin or sulfonylureas)
-
Pancreatitis
-
Allergic reactions
Seek immediate medical attention if severe reactions occur.
Precautions and Warnings
-
Prescription medicine; use only under a doctor’s supervision
-
Not indicated for Type 1 diabetes
-
Use caution in patients with gastrointestinal disorders
-
Inform your doctor about all ongoing medications
-
Not recommended during pregnancy or breastfeeding unless advised
-
Do not share the pen between patients
Storage Instructions
-
Store refrigerated at 2°C to 8°C
-
Do not freeze
-
Protect from light
-
Follow storage instructions mentioned in the package insert after first use
Key Highlights
-
Contains Tirzepatide 2.5 mg
-
Dual GIP & GLP-1 receptor agonist
-
Once-weekly injection for better compliance
-
Easy-to-use prefilled KwikPen
-
Manufactured by Eli Lilly, a globally trusted pharmaceutical company








Reviews
There are no reviews yet.